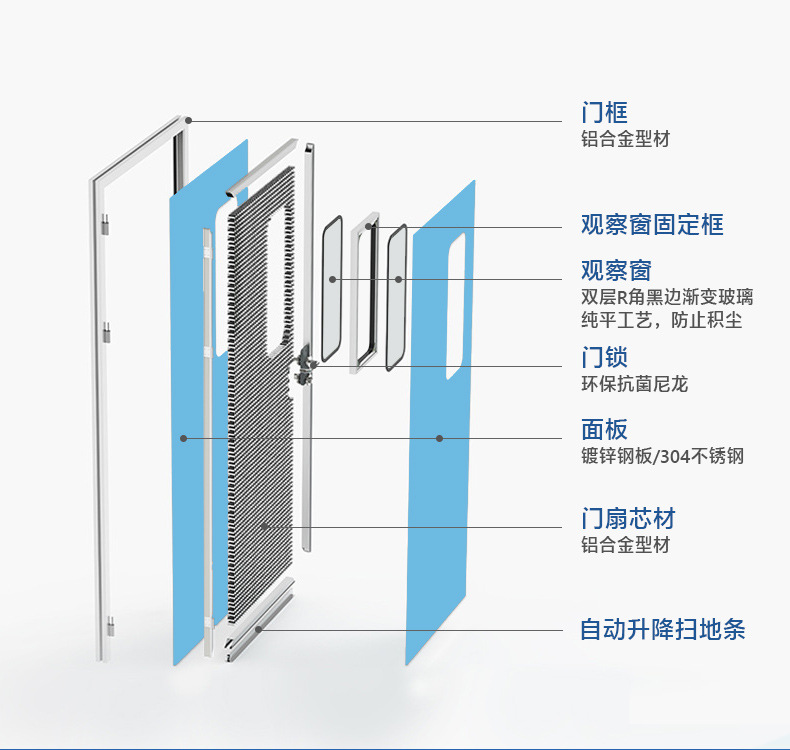
When designing medical airtight door bodies, the door frame should be selected to ensure sufficient rigidity requirements, and a certain thickness of angle steel should be selected during design; When selecting door leaf materials, consider the anti-corrosion measures inside the door body and the stiffness of the door leaf. Choose a certain thickness of galvanized steel plate for bending and forming, which not only solves the overall flatness and stiffness of the door leaf, but also meets the requirements of the closed gap between the door leaf and the door frame, so that the gap of the medical airtight door is controlled within 1mm after closing.
 To ensure the sealing effect when the medical airtight door is closed, the gap size of the door leaf is determined based on the compression data of the selected sealing material, and a square sealing material with moderate compressibility is selected to be placed around the door and easy to replace. EPDM rubber is used as the sealing material, which has the characteristics of low density, low cost, good chemical resistance, and good aging resistance. The test shows that this specification and the sealing material of this variety fully comply with the requirements of the "Technical Specification for Design of Medical Airtight Doors". The accessories used for medical watertight doors should not only meet the corresponding performance requirements, but also have sealing performance. For example, the pressure relief device should have a handwheel that can be operated both inside and outside, and turning the handwheel can relieve pressure. The pressure relief device should be sealed when tightening the handwheel and can withstand a working pressure of 5000Pa. The medical waterproof door lock device also has the function of being operable both inside and outside. When the handle is locked, the handle shaft can rotate and seal, supporting a working pressure of 5000Pa. In this way, the design of pressure reducing valves and locking handles, as well as the selection of sealing materials, have become the research focus of medical airtight doors.
To ensure the sealing effect when the medical airtight door is closed, the gap size of the door leaf is determined based on the compression data of the selected sealing material, and a square sealing material with moderate compressibility is selected to be placed around the door and easy to replace. EPDM rubber is used as the sealing material, which has the characteristics of low density, low cost, good chemical resistance, and good aging resistance. The test shows that this specification and the sealing material of this variety fully comply with the requirements of the "Technical Specification for Design of Medical Airtight Doors". The accessories used for medical watertight doors should not only meet the corresponding performance requirements, but also have sealing performance. For example, the pressure relief device should have a handwheel that can be operated both inside and outside, and turning the handwheel can relieve pressure. The pressure relief device should be sealed when tightening the handwheel and can withstand a working pressure of 5000Pa. The medical waterproof door lock device also has the function of being operable both inside and outside. When the handle is locked, the handle shaft can rotate and seal, supporting a working pressure of 5000Pa. In this way, the design of pressure reducing valves and locking handles, as well as the selection of sealing materials, have become the research focus of medical airtight doors.
 According to the technical specifications for the design of medical airtight doors and the requirements for inspecting key areas, the medical door manufacturer has designed and drawn a complete set of drawings, locks, and processing for the pressure reducing valve and pressure reducing handle, and determined the sealing material. The size of the sealing component and the trial production product test fully meet the design requirements.
According to the technical specifications for the design of medical airtight doors and the requirements for inspecting key areas, the medical door manufacturer has designed and drawn a complete set of drawings, locks, and processing for the pressure reducing valve and pressure reducing handle, and determined the sealing material. The size of the sealing component and the trial production product test fully meet the design requirements.